Our Science
We Are Experts in Lysosomal Enzymes.
Our team is the first to unlock the potential of mannose 6-phosphate (M6P) to improve and expand treatments for individuals with lysosomal storage disorders (LSDs), a family of more than 50 rare inherited diseases. We are translating science into valuable investigational therapies – the next generation enzyme replacement and gene therapies for LSDs – with the potential to relieve suffering, restore health, and improve lives.
M6P is a specialized carbohydrate structure that is the natural biological signal that cells use to identify lysosomal enzymes and for transporting them to lysosomes. In order to exert their therapeutic effect, the therapeutic lysosomal enzymes have to bind receptors on cell surfaces for entering cells and subsequent delivery to the lysosome. We do this by harnessing the naturally existing M6P receptor targeting pathway that is present on most if not all cells, which helps to optimize the biodistribution and lysosomal uptake of ERT and the efficient cross-correction of gene therapy products. With our innovative S1S3 co-expression platform technology, we are able to increase the levels of M6P on therapeutic lysosomal enzymes thereby improving their ability to bind M6P receptors and be taken up into cells and organs lacking sufficient therapeutic enzyme. With our platform, we have the capability to develop better targeted ERTs and gene therapy products.

What's the Problem?

The Breakthrough Discovery: S1S3 Variant of GlcNAc-1-phosphotransferase
Phosphorylation is a process in which a phosphate group is added to a molecule, such as a sugar or a protein. The phosphorylation of terminal mannose sugar residues on newly synthesized lysosomal enzymes is performed by GlcNAc1-phosphotransferase (PTase) to form M6P is very different than phosphorylation of certain amino acids on proteins by kinases: the former is utilized for the transport of lysosomal enzymes to lysosomes; the latter phosphorylation process is utilized by cells to activate or inactivate various proteins akin to the function of light switches. Although scientists have understood the process for phosphorylating lysosomal enzymes for decades, Drs Stuart Kornfeld and Lin Liu from Washington University, St. Louis, MO, were the first to figure out how to modify GlcNAc-1-phosphotransferase to enhance its enzymatic activity and reliably increase M6P levels on lysosomal enzymes thereby harness the potential for better cellular uptake and delivery of therapeutic enzymes.
Drs Kornfeld and Liu achieved the breakthrough when they created a truncated, pre-activated variant of GlcNAc-1-phosphotransferase — the S1S3 PTase variant. With this variant, they demonstrated that they can efficiently phosphorylate all soluble lysosomal enzymes that they tested.
In a preclinical cellular model, when Drs Kornfeld and Liu added the DNA sequences for expression of both the S1S3 PTase variant and the various lysosomal enzymes, the cells were able to produce the lysosomal enzymes with high M6P content. The produced lysosomal enzymes with high M6P content were shown to have substantially higher binding affinity for the M6P receptor that is present on cell surfaces and utilized for cellular uptake.
Dr. Kornfeld and Liu published their results in Mol Ther Methods Clin Dev demonstrating that the S1S3 variant can enhance the M6P content of lysosomal enzymes and that the resulting lysosomal enzymes are better able to bind to cell surface receptors.
With our platform technology, we are able to control this process for the first time and increase levels of M6P on receptor cells.
GlcNAc-1-Phosphotransferase adds M6P to Lysosomal Enzymes
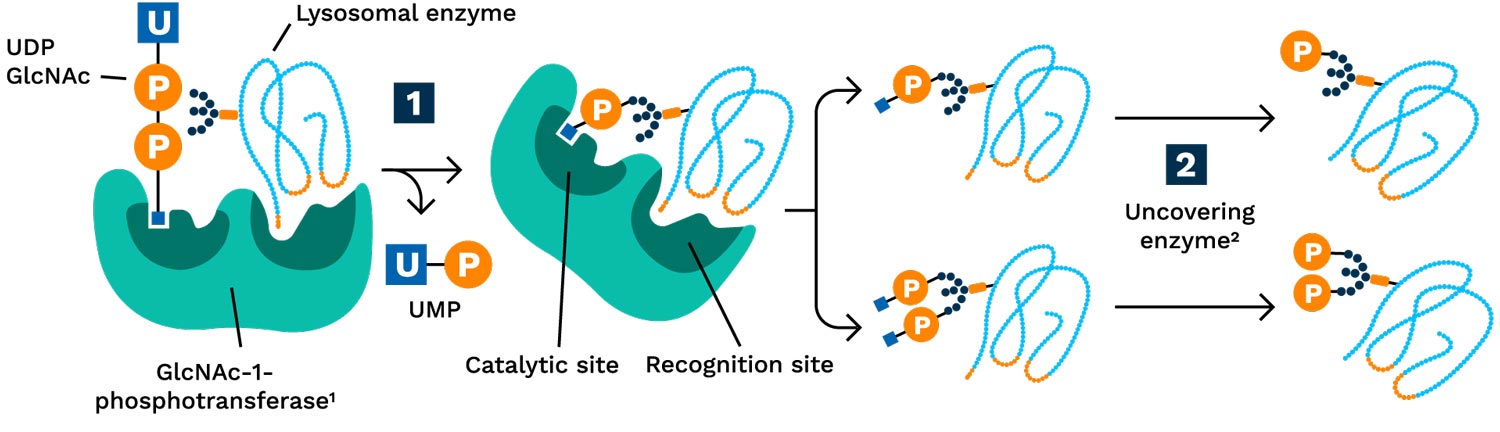
1GlcNAc-1-phosphotransferase catalyzes the transfer of GlcNAc-1-phosphate from uridine diphosphate (UDP) onto terminal mannose residues of the N-linked oligosaccharides on enzymes destined for the lysosome.
2 NAGPA (N-acetylglucosamine-1-phosphodiester alpha-N-acetylglucosaminidase), also known as uncovering enzyme, removes covering GlcNAc group, thereby exposing mannose-6-phosphate (M6P).

