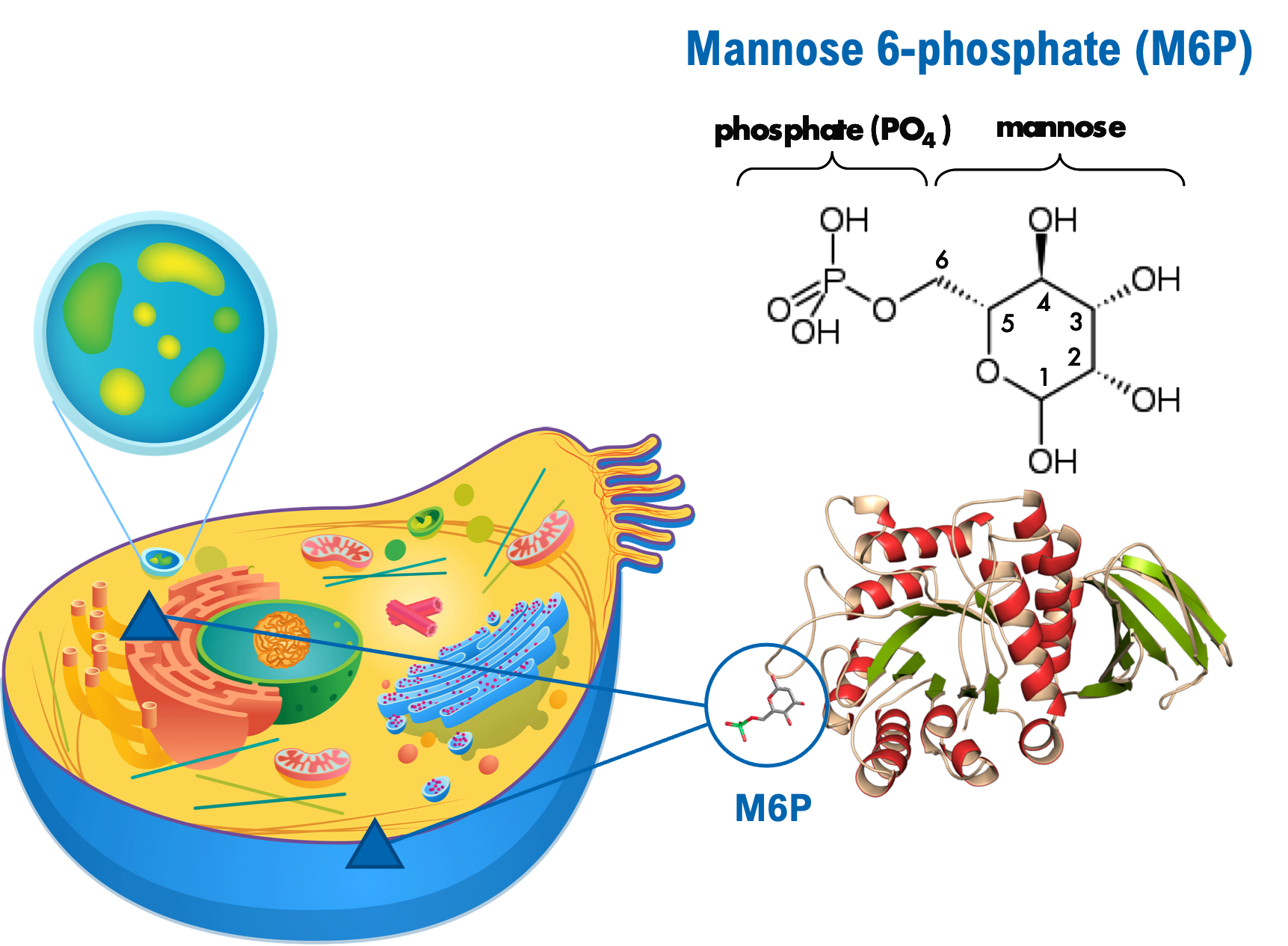
Mannose 6-Phosphate (M6P)
We Harness the Potential of Mannose 6-Phosphate (M6P).
Mannose 6-phosphate (M6P) is a specialized carbohydrate structure found on lysosomal enzymes. M6P enables newly synthesized lysosomal enzymes to bind to M6P receptors in Golgi for delivery of the lysosomal enzymes to lysosome within cells. There are also M6P receptors on the cell surface of most, if not all cells, that can bind exogenous M6P-bearing lysosomal enzymes to enable their cellular uptake and delivery to lysosomes. It is this natural receptor-mediated cellular uptake process that is utilized by therapeutic lysosomal enzyme treatments for LSDs.
Membrane-bound M6P receptors are the native pathway for transporting lysosomal proteins to lysosomes. With low levels of M6P, the enzymes are not able to reach the lysosomes as effectively as they are if the levels are higher.
A current limitation for the majority of enzyme replacement therapies (ERTs) is that they lack sufficient amounts of M6P to allow for efficient cellular uptake via the M6P receptor pathway. Most current and potential treatments, including gene therapies, cannot naturally increase M6P levels which limits their biodistribution and cellular uptake in affected cells and tissues. Earlier efforts to enhance M6P content on the produced lysosomal enzymes were not successful.
Cation-Independent Mannose 6-Phosphate Receptor (CI-MPR)
Receptor responsible for trafficking phosphorylated (M6P targeted) enzymes to lysosomes
Source: Modified from https://mrmitchellsbiology.weebly.com
Meeting the Historic Challenge
Historically, it has been difficult to create ERTs with high amounts of M6P – no one has been able to reliably increase the content of M6P on lysosomal enzymes. From a gene therapy perspective, this approach has been shown to produce high amounts of therapeutic lysosomal enzymes, but it cannot improve the endogenous phosphorylation process. Thus, it is virtually impossible to control carbohydrate processing in vivo to increase M6P on gene products.
Earlier efforts to enhance M6P content have largely failed despite decades of effort. Consequently, most ERTs and gene therapy products do not reach the lysosomes in affected cells, where they are needed, in sufficient quantities for optimal clinical impact.
How Do We Solve the Challenge?
With our S1S3 co-expression platform technology, we have the capability of increasing cells’ ability to phosphorylate lysosomal enzymes to reliably and naturally increase M6P content. This same technology can be applied for both ERT and for gene therapy.
We engineer our ERTs to have high amounts of M6P, which enable better targeting to lysosomes, which can result in increased efficacy across more tissue types and potential first-in-class therapies for untreated diseases and best-in-class therapies for diseases with a treatment option.
Our initial research programs are focused on Pompe disease, Gaucher disease, Fabry disease, and mucolipidosis II (ML II), and we plan to initiate our first clinical program in 2023.

